Making A Difference Through Optimizing Cyclosporine Treatment With DoseMe
In this article:
THE CUSTOMER
Maribor University Medical Centre is one of the largest medical institutions in Slovenia. It is a public health care institution performing healthcare services on secondary and tertiary level for the wider region of Maribor, Pesnica, Ruše, Ormož, Lenart, Ptuj and Slovenska Bistrica.
THE CHALLENGE
Despite the emergence of new drugs, cyclosporine remains a critically important immunosuppressant. Therapeutic drug monitoring (TDM) of immunosuppressive therapy with cyclosporine is a critical requirement because of its narrow therapeutic index and significant inter-individual variability in blood concentrations.1,2
Dose individualization of cyclosporine was performed by taking two blood samples at fixed times – a pre-dose trough (C0) and two hours post administration (C2) – to interpret results into a dose recommendation.1,6
This method is associated with a number of challenges in clinical practice, including difficulties drawing two blood samples. There is ongoing discussion as to whether C0-only or C2-only monitoring is sufficient.2,3
 KEY BENEFITS
KEY BENEFITS
- DoseMe® simplified the ability of UKC practitioners to optimize each patient’s cyclosporine therapy in this study.
- TDM using only C0 measurement
is sufficient and results in the same dosing recommendation as when using both C0 and C2 measurements in autoimmune patients utilizing DoseMe.
 CUSTOMER
CUSTOMER
Maribor University Medical Centre
 LOCATION
LOCATION
Maribor, Slovenia
 WEBSITE
WEBSITE
 ORGANIZATION TYPE
ORGANIZATION TYPE
Public Hospital
 BEDS
BEDS
1,226
 INPATIENT VISITS PER YEAR
INPATIENT VISITS PER YEAR
60,000
 PRODUCT IMPLEMENTED
PRODUCT IMPLEMENTED
DoseMe®
THE SOLUTION
A prospective study was initiated by UKC to determine the appropriateness of pharmacokinetic software DoseMe to optimize treatment of cyclosporine patients, as well as determine if monitoring only pre-dose trough (C0) blood levels results in the same dosing recommendation as when using both C0 and C2 measurements for autoimmune diseases.
Study Design
Eleven patients at University Medical Centre Maribor (UKC) in Maribor, Slovenia with autoimmune diseases were treated with cyclosporine.
UKC monitored cyclosporine blood concentrations for each patient and optimized their treatment, based on the measured value. Whenever possible, both C0 and C2 blood samples were taken.
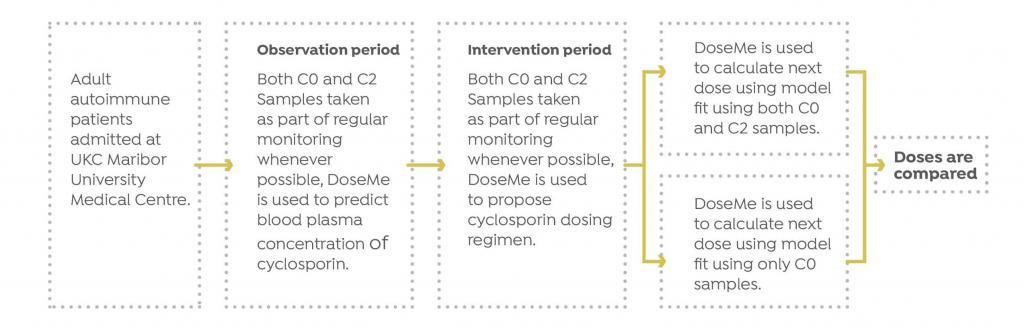
The Results
DoseMe demonstrated that TDM using only a single C0 measurement is sufficient, resulting in the same dosing recommendation using both C0 and C2 measurements in autoimmune patients. Dose recommendations were calculated using DoseMe individualized models fitted to only C0 values, and to both C0 and C2 values for eight of eleven patients.
TABLE 1
Summary of dose recommendation taking into account only the C0 and C0 and C2 sample
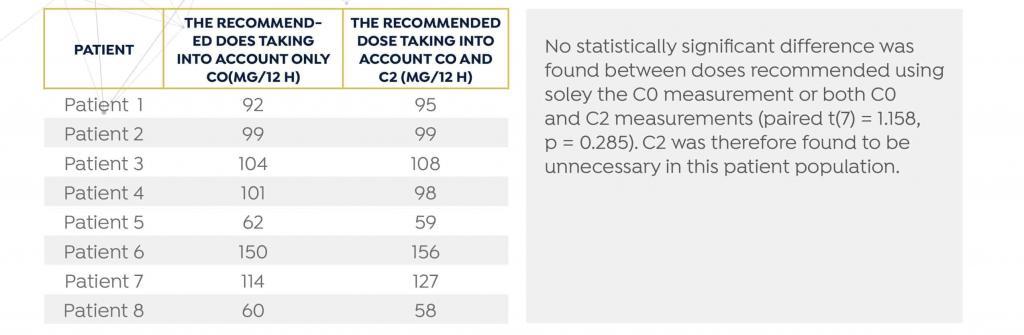
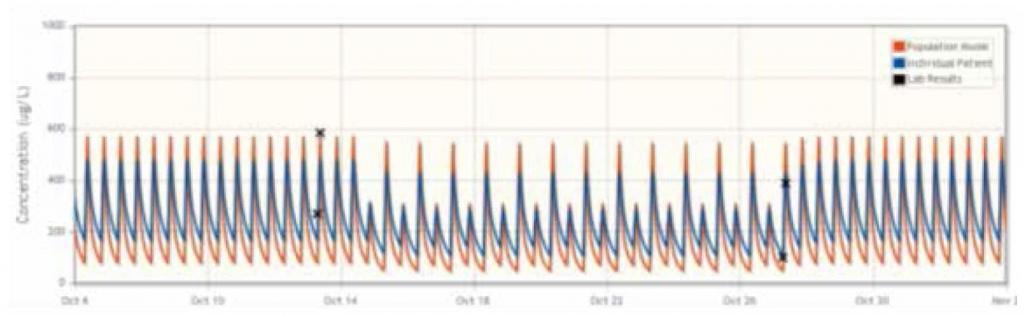
FIGURE 1
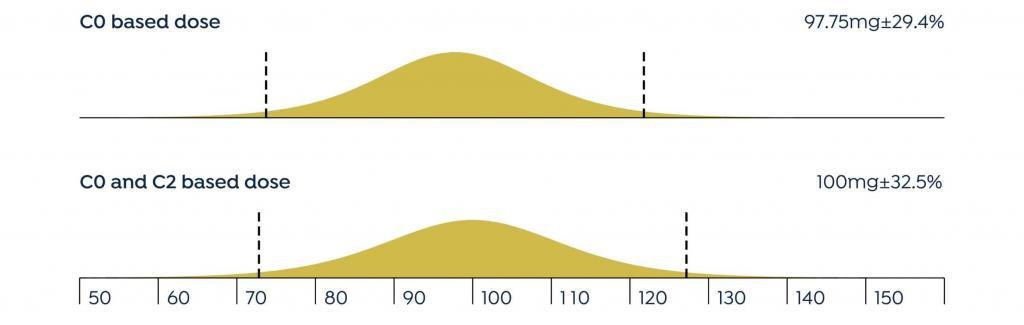
The mean difference between the doses recommended by DoseMe using only C0 and both C0 and C2 is statistically indistinguishable from zero:
In the majority of cases, UKC Maribor found that the DoseMe software adapted the population cyclosporine pharmacokinetic model to individual patients, as well as accurately simulated the patient’s blood plasma concentration profile. DoseMe effectively recommended a clinically relevant dosing regimen aimed at keeping the blood plasma concentration in the drug’s therapeutic range. In seven of eleven cases, DoseMe’s recommended dosing regimen was accepted by the practitioners. In two of these cases, the practitioners opted to split the total daily dose in different proportion between the morning and afternoon doses.
In two cases, the treatment was discontinued due to adverse side effects. DoseMe was effective at predicting blood plasma concentration of cyclosporine in both of those cases, with DoseMe’s calculation demonstrating that only a higher than tolerated dose of cyclosporine would result in concentration reaching therapeutic range, supporting the decision to discontinue of treatment with cyclosporine.
In all but two cases, the software advice was accepted by clinicians. In one case, DoseMe’s recommendation to raise the dose was not accepted by the clinician as the patient’s condition was already stable. In the second case, DoseMe’s recommendation to raise dose was not accepted and treatment was discounted as ineffective, possibly due to sub-therapeutic blood plasma concentration.
Patient Case Studies
Intentionally Introducing Dilitazem
The clinician intentionally introduced dilitiazem concomitantly with cyclosporine in order to increase blood levels of cyclosporine at lower doses5 , as well as reduce proteinuria in a glomerulonephritic patient. DoseMe was able to fit the population model to a patient with glomerulonephritis, and a dose that resulted in cyclosporine concentration in the middle of therapeutic range was proposed and accepted by the clinician, successfully adapting the treatment to this non-typical patient.
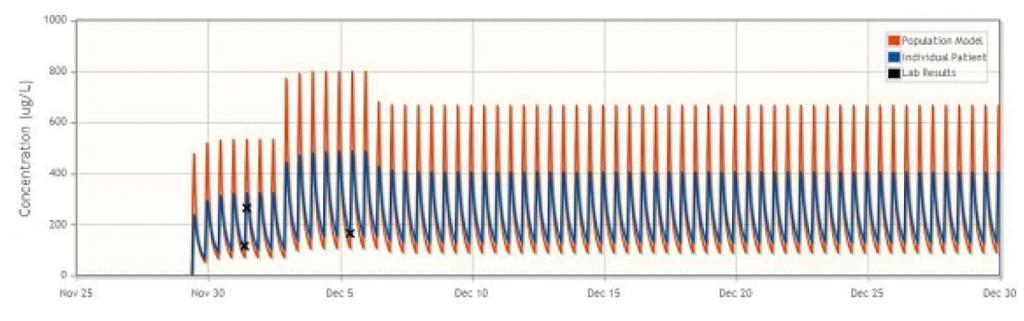
Aplastic Anaemia: Suspected Drug Interaction
During the treatment of a patient with aplastic anaemia, a sudden increase in cyclosporine concentration was observed despite the unchanged dosing regimen, possibly due to a suspected drug interaction. DoseMe proposed a reduction in daily dose, and after 14 days a lowering of cyclosporine blood plasma concentration was observed. DoseMe was then used to propose a higher dose, which was subsequently adopted. The patient did not observe adverse effects in the treatment, and the results of laboratory tests were within the normal range. DoseMe enabled adequate management of this patient’s treatment despite complications.
Conclusion
DoseMe pharmacokinetic software has proven to be a useful instrument for optimization of treatment with cyclosporine in a demanding clinical setting at UKC Maribor. This included supporting treating physicians in determining the optimum dosage regimen for individual patients and assisting decisions whether to continue or cease cyclosporine treatment.
About DoseMe
DoseMe is the first company in the world to offer precision dosing software specifically developed for clinical use at the point-of-care. The DoseMe platform significantly improves dosing accuracy and patient outcomes by providing real-time dose-related decision support to enable precision medicine using readily available patient data.
Print this whitepaper
REFERENCES
1. Novartis Pharmaceuticals Corporation. NEORAL Prescribing Information. 2015.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050715s035,050716s038lbl.pdf
2. Hermann M, Enseleit F, Fisler AE, Flammer A, Lüscher TF, Noll G, Ruschitzka F. Cyclosporine C0- versus C2-monitoring over three years in maintenance heart transplantation. Swiss Med Wkly. 2011 Feb 3;141:w13149. doi: 10.4414/smw.2011.13149.
3. Kyllönen LE, Salmela KT. Early cyclosporine C0 and C2 monitoring in de novo kidney transplant patients: a prospective randomized single-center pilot study. Transplantation. 2006 Apr 15;81(7):1010-5.
4. Stopinšek M. Optimisation of cyclosporine treatment at University Medical Centre Maribor. [Masters Thesis]. Ljubljana. 2017.
5. Xue W, Song Y, Tian P, Ding X, Pan X, Yan H, Hou J, Feng X, Xiang H, Tian X. The effects of diltiazem in renal transplantation patients treated with cyclosporine A. J Biomed Res. 2010 Jul;24(4):317-23. doi:10.1016/S1674-8301(10)60044-9.
Try DoseMeRx – Free Trial
Try DoseMeRx for yourself, with a free 14 day trial including your choice of drug packages from a wide range of medications, customize dose or target functions to individualize treatment for every patient, and access our mobile and tablet apps for iOS and Android. During your free trial, you’ll also have access to our support team who are here to answer any questions you have. Start free trial now >>

 KEY BENEFITS
KEY BENEFITS CUSTOMER
CUSTOMER LOCATION
LOCATION WEBSITE
WEBSITE ORGANIZATION TYPE
ORGANIZATION TYPE BEDS
BEDS INPATIENT VISITS PER YEAR
INPATIENT VISITS PER YEAR PRODUCT IMPLEMENTED
PRODUCT IMPLEMENTED